On December 23, 2019, a paper entitled “Differential White Matter Maturation from Birth to 8 Years of Age” was online published in Cerebral Cortex by Prof.Fang Fang’s group at the School of Psychological and Cognitive Sciences at Peking University, Peking-Tsinghua Center for Life Sciences and the PKU-IDG/McGovern Institute for Brain Research, and Prof.Hao Huang’s group at the University of Pennsylvania. This study acquired diffusion tensor imaging data from 118 typically developing children aged 0 to 8 years and 31 children with autism aged 2 to 7 years. Modelling with exponential function, the maturation of white matter was found spatially and temporally differential. The white matter maturation was further separated into fast, intermediate, and slow phase according to the developmental rate. This study provides a standard development reference, which can serve as a biomarker for early detection of neuropsychiatric disorders.
During infancy and childhood, the human brain white matter undergoes significant microstructural changes with dynamic axonal and myelin maturation. These white matter maturational processes underlie the structural basis of emerging brain circuits critical to memory, attention, intelligence, language, motor learning, musical proficiency, and cognitive control. Dysfunction of the white matter maturational processes is associated with a suite of neuropsychological disorders including schizophrenia, major depressive disorder, bipolar disorder, autism, and attention-deficit hyperactivity disorder (ADHD). Delineation of maturational white matter microstructural curves of all major white matter tracts and tract groups of typically developing (TD) brain could not only reveal the spatiotemporal differential circuit formation in normal brain development, but also set the stage for understanding aberrant brain development in neurodevelopmental disorders such as autistic spectrum disorder (ASD) and developmental brain disorders in general.
Diffusion MRI, especially diffusion tensor imaging (DTI), has been widely used to quantify white matter microstructure. Water molecules tend to diffuse more freely along the white matter tracts, rather than perpendicular to the tracts. These diffusion properties of water molecules around white matter tracts can be measured through DTI, which uses a tensor model to measure the water diffusion in vivo. DTI-derived metrics are sensitive to white matter microstructural changes during development. Fractional anisotropy (FA), ranging from 0 to 1, has been utilized to quantify the shape of the diffusion tensor. Radial diffusivity (RD) and axial diffusivity (AD), the diffusivity measurements perpendicular to and along the diffusion tensor, are associated with myelination and axonal growth, respectively. Mean diffusivity (MD) quantifying the size of the diffusion tensor usually decreases during brain development. In white matter, the microstructural maturation, including axonal growth, axonal packing, and fiber myelination, can be quantified by the changes of the four DTI-derived metrics (Fig. 1).
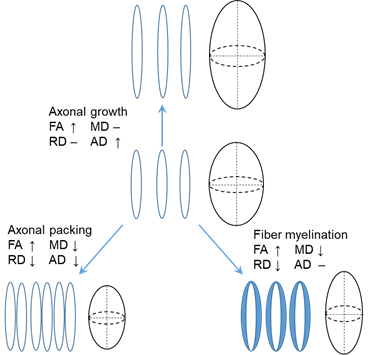
Fig 1, Changes of FA, MD, RD, and AD caused by axonal growth, axonal packing and fiber myelination.
In previous studies, insufficient sample covering the critical development period from birth to early childhood brings enormous difficulty to quantify white matter development. To cover the deficiencies, we acquired DTI data from a larger sample size, 118 typically developing (TD) children, at the age with critical development, which is from 0 to 8 years. Fig. 2 illustrates the DTI metrics changes in the white matter from 2 to 95 months. At this period, the brain volume increased, white matter FA increase, MD and RD decreased.
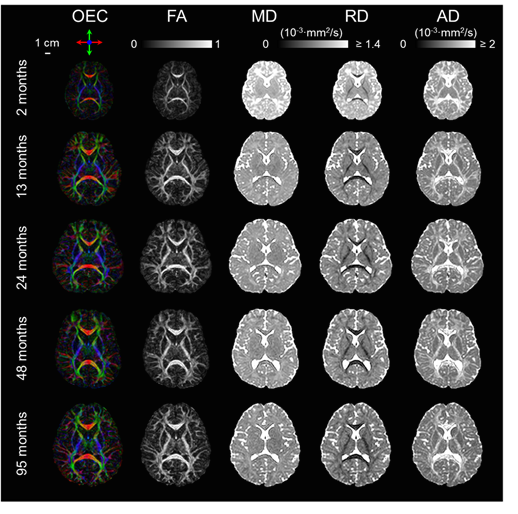
Fig. 2, Changes of orientation-encoded colormap (OEC), FA, MD, RD, and AD from 2 to 95 months
For quantification white matter microstructure across subjects, the FA images from all subjects were registered to the white matter atlas JHU ICBM-DTI-81 (Mori et al. 2008). The white matter skeleton was then extracted using tract-based spatial statistics (TBSS) (Fig. 3A). white matter tracts are categorized into commissural (interhemispheric connection), brainstem (connectivity in brainstem and cerebellum), association (corticocortical connections), limbic (connectivity in limbic system), and projection (corticospinal connections) tract groups based on the tract functions (Wakana et al. 2004) (Fig. 3B). The labels of the white matter tracts were transferred from the atlas to the white matter skeleton (Fig. 3C).

Fig. 3, Registering FA images to the atlas, extracting white matter skeleton (A), and then separating white matter into different tracts and tract groups (B & C).
To find out the optimal white matter development model, the FA, MD, RD, and AD of the entire white matter of the 118 subjects were fitted with five candidate models, including linear, logarithmic, exponential, Poisson, and quadratic polynomial models, respectively (Fig. 4). The exponential model popped out with the highest goodness-of-fit (all higher than 76.8% for four DTI metrics) among five models. The Akaike information criterion result showed the exponential model had more than 99% probability to be the optimal model. Hence, the exponential model was applied to characterize white matter maturation.
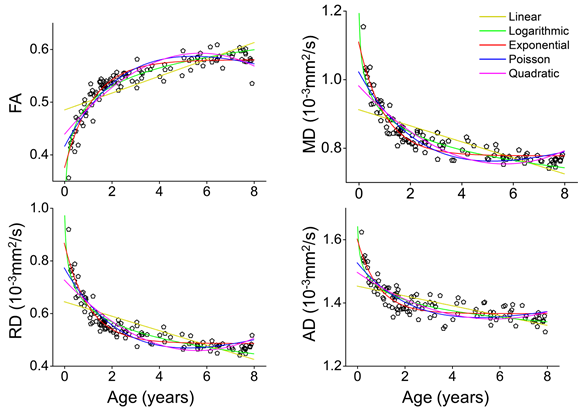
Fig. 4, fitting FA, MD, RD, and AD of the entire white matter with five candidate models.
Next, we fitted the DTI metrics changes of the white matter tracts and tract groups with exponential model. Fig. 5 shows the maturation of each white matter tract from 0 to 8 years, companied with exponential fitted curve. We found that FA of all tracts increased nonlinearly, and RD, MD, and AD of most tracts decreased nonlinearly during development.
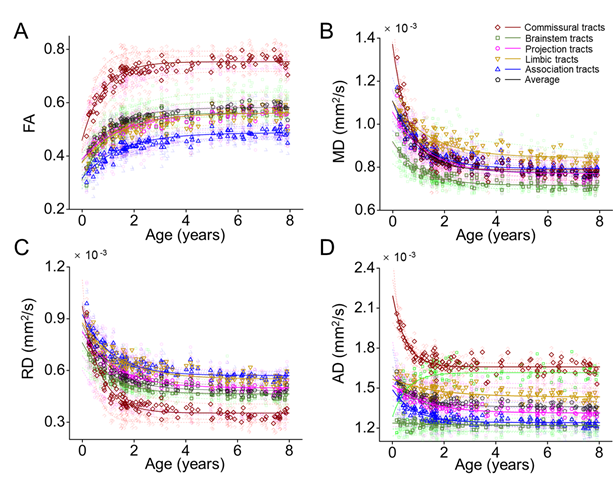
Fig. 5, FA of white matter tracts increased, MD, RD, and AD of white matter tracts decreased during development. The tendencies of the changes of the DTI metrics by ages are exponential. The developmental rates of white matter tracts are differential.
Further, to discriminate developmental rate between white matter tracts, two time points reaching the 2/3 and 8/9 of the increase of the fitted FA from 0 to 8 years were identified to separate the development into three phases, namely fast, intermediate, and slow phase. Fig. 6 shows the periods of the three phases are different among the five tract groups and the entire white matter. The commissural tracts matured earlier with shorter fast phase and longer slow phase. The association tracts, which are highly related to cognitive function, matured later with later ending of fast phase and later starting of slow phase.
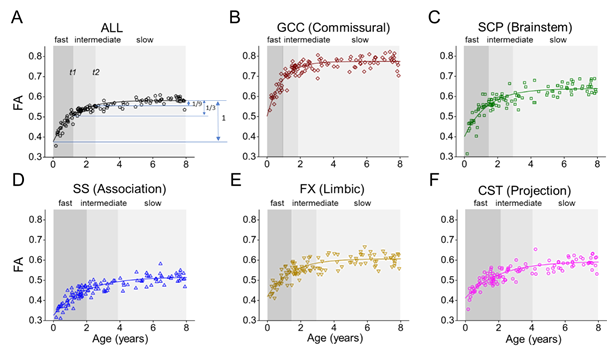
Fig. 6, fast, intermediate, and slow phase of white matter development.
The white matter development can be characterized and quantified by the parameters from the exponential model f(x)=a·e-bx+c. In FA, the absolute value of parameter a is in direct proportion to the amplitude of development f(x)-f(0)= a·(e-bx-1); parameter b modulates the developmental rate, which is inversely proportional to the age x to reach the same maturation level f(x). The formula can be transformed to bx=-log((f(x)-c)/a); parameter c is a constant, indicates the baseline of the developmental curve (Fig. 7).
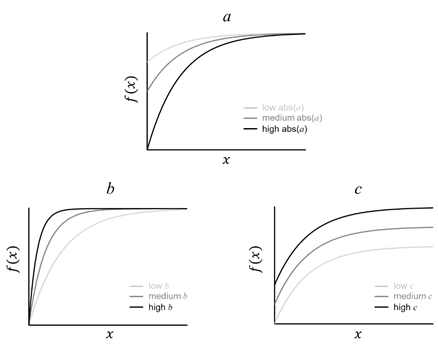
Fig. 7, Parameters a, b, and c from the exponential model f(x)=a·e-bx+c modulate the amplitude, rate, and baseline of development, respectively.
Three phases and three parameters were then estimated in the four DTI metrics of each white matter tract. In FA, compared with the entire white matter, the commissural tracts matured with shorter fast phase, longer slow phase, higher amplitude (a), rate (b), and baseline (c), while the association tracts matured with later ending of fast phase, later starting of slow phase, lower amplitude (a), rate (b), and baseline (c) (Fig. 8). Two general white matter maturation patterns could be identified with the fitted parameters shown in Fig. 8: 1) white matter develops from the posterior to anterior brain. For example, the splenium of corpus callosum (SCC), posterior portion of internal capsule (PIC), and posterior portion of corona radiata (PCR) mature earlier than the genu of corpus callosum (GCC), anterior portion of internal capsule (AIC), and anterior portion of corona radiata (ACR), respectively; 2) white matter develops from the central to peripheral brain. For example, the central commissural tract group matures earlier than the peripheral association tract group. The white matter tracts connected to the primary sensory cortex matures earlier than the tracts connected to the cortex associated with higher cognitive function. For instance, the SCC, which connected the inter-hemispheric primary visual cortex, matures earlier than the superior longitudinal fasciculus (SLF) and superior fronto-occipital fasciculus (SFOF), both connected to the prefrontal cortex.
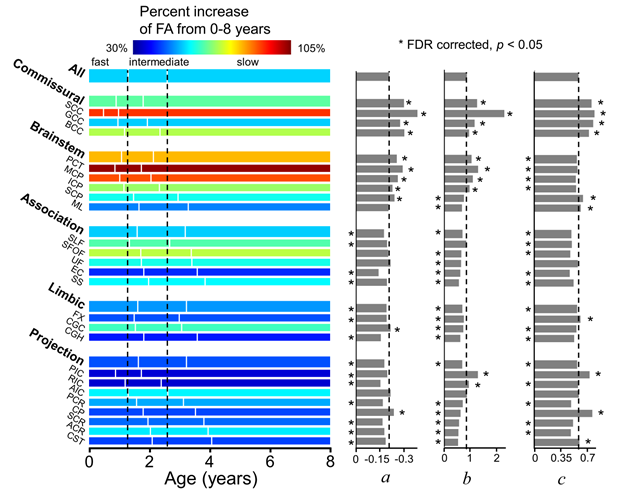
Fig. 8, Three phases and three parameters are differential among white matter tracts.
Abnormality is defined if and only if normality is defined. The typical white matter developmental track of white matter delineated in current study will help to detect aberrant brain development associated with neuropsychiatric disorders as earlier as possible, allowing for early intervention and precision health for infants and children. Comparing with the maturation of white matter from 31 children with autism aged 2 to 7 years, the residual variance of FA in children with autism was found higher than in TD subject in the commissural, limbic tracts and entire white matter (Fig. 9). The larger inter-individual variability of white matter microstructural may be the neural basis of higher behavior variability in children with autism (Humphreys et al.2007; Turner and Stone 2007).
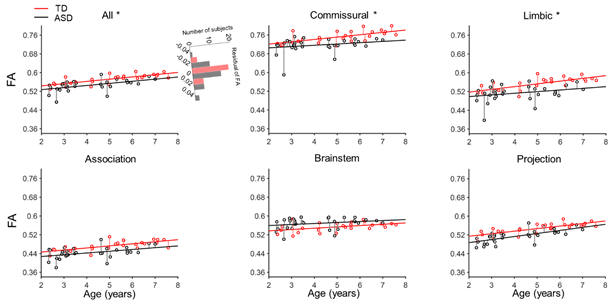
Fig. 9, The residual variance of the FA of children with autism (black) was found significantly larger than the residual variance of TD children (red) in the commissural, limbic, and entire white matter.
This study was supported by the National Institutes of Health (NIH), National Natural Scientific Foundation of China (NSFC), the Ministry of Science and Technology of China (MOST), and the Peking-Tsinghua Center of Life Sciences (CLS). The corresponding authors are Prof. Fang Fang and Prof. Hao Huang. The first authors are Dr. Qinlin Yu, a graduated Ph.D. from CLS and Prof. Yun Peng from Capital Medical University.
Link of the paper:https://doi.org/10.1093/cercor/bhz268
Reference:
Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R. 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template.Neuroimage. 40:570–582.
Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. 2004. Fiber tract–based atlas of human white matter anatomy. Radiology. 230:77–87.
Humphreys K, Minshew N, Leonard GL, Behrmann M. 2007. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 45:685–695.
Turner LM, Stone WL. 2007. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology and Psychiatry. 48:793–802.


